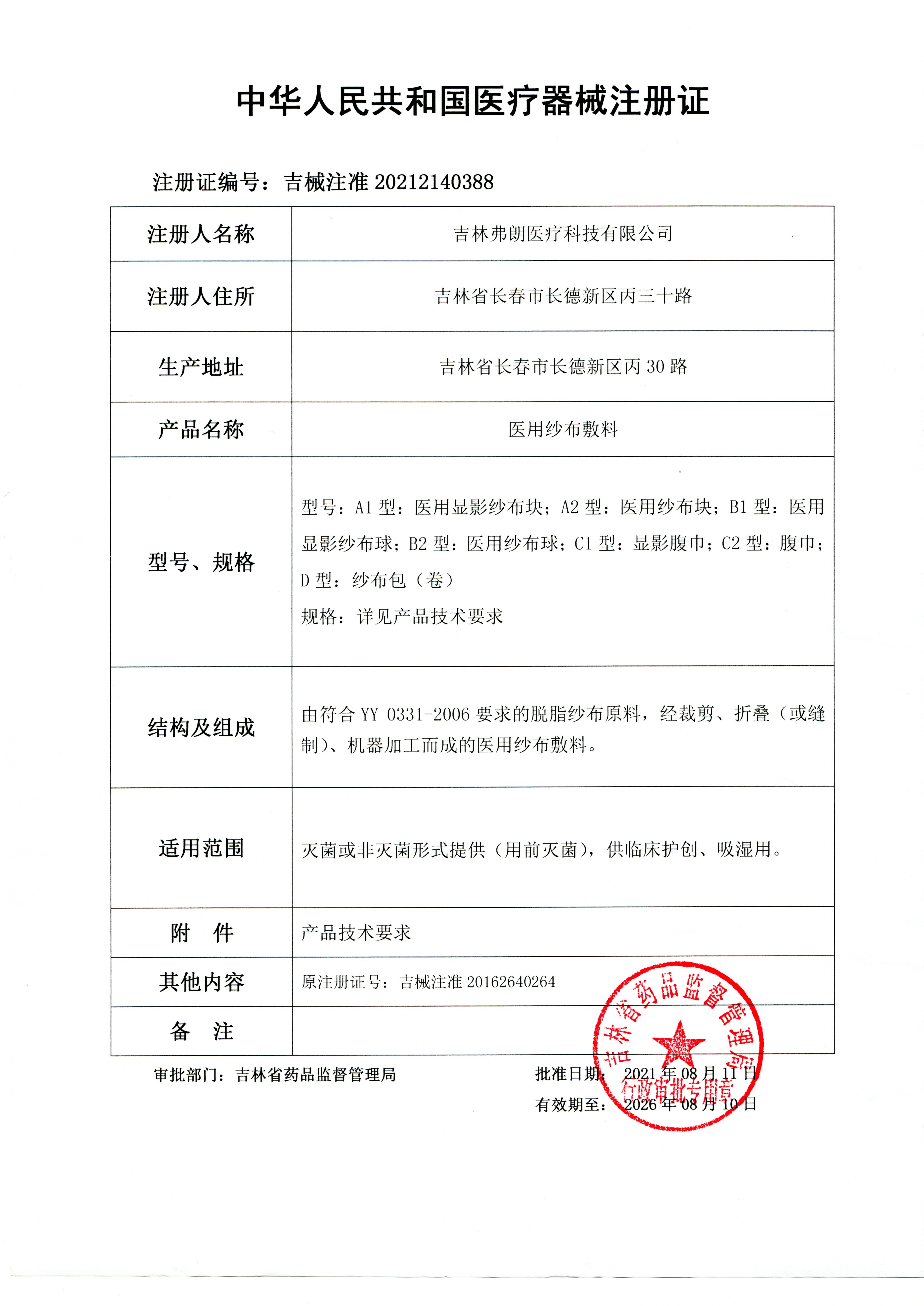
[Product Name] Medical gauze dressing
[Specification and Model] Model: A1 type: Medical developing gauze block; A2 type: medical gauze block
[Registrant/Company Name] Jilin Fran Medical Technology Co., Ltd
[Domicile] Bing30th Road, Changde New District, Changchun, Jilin Province
[Production address] Bing30 Road, Changde New District, Changchun, Jilin Province
[Contact Information] 0431-81851702
[Production License No.] Ji Shi Yao Jian Xi Production License No. 20150048
[Product Registration Certificate Number] Jixi Registration Number: 20162640264
[Product Technical Requirements No.] Jixi Registration No.: 20162640264
The main structure of the product is a medical gauze dressing made from skimmed gauze raw materials that meet the requirements of YY 0331-2006, which are cut, folded (or sewn), and machine processed.
[Product performance] 1. Basic dimensions: meet the technical requirements of the product
2. Appearance: The surface should be clean, free from stains, holes, and other defects.
3. Folding and Sewing: The folding or sewing method should ensure that the cut edges of the gauze do not leak out, the sewing should be firm, the sewing thread should be straight, and there should be no skipping or needle jumping.
4. The raw materials for gauze should meet the requirements of YY 0331-2006.
5. X-ray detectable components
5.1 X-ray detectable components should be made of materials with a content of not less than 55% barium sulfate or other materials with equivalent X-ray opacity. This material should not shed fibers and should not affect the softness of the dressing.
5.2 The quality of X-ray detectable components should not be less than 0.5g/m.
5.3 X-ray opacity
6. Sutures
6.1 Fluorescent substances
6.1.1 When testing cotton sutures according to Test A in Appendix D of YY0594-2006, only microscopic brownish purple fluorescence and a small amount of yellow particles should be observed. Except for a small amount of isolated fibers, strong blue fluorescence should not be observed.
6.1.2 Polymer sutures and abdominal bandages should meet the requirements when tested according to Appendix D of YY0594-2006.
6.2 The total amount of soluble substances in water shall not exceed 0.50%.
7. Abdominal bandage
7.1 Material: The abdominal scarf should be sewn with cotton yarn and/or adhesive yarn tightly woven fabric. The length should be ≥ 5cm.
7.2 Fluorescent material: When testing the abdominal bandage fabric according to Test A in Appendix D of YY0594-2006, only micro brown purple fluorescence and a small amount of yellow particles should be observed