[Product Name] Disposable Medical Hat
[Specification and model] (340 ± 10) mm × (155 ± 5) mm
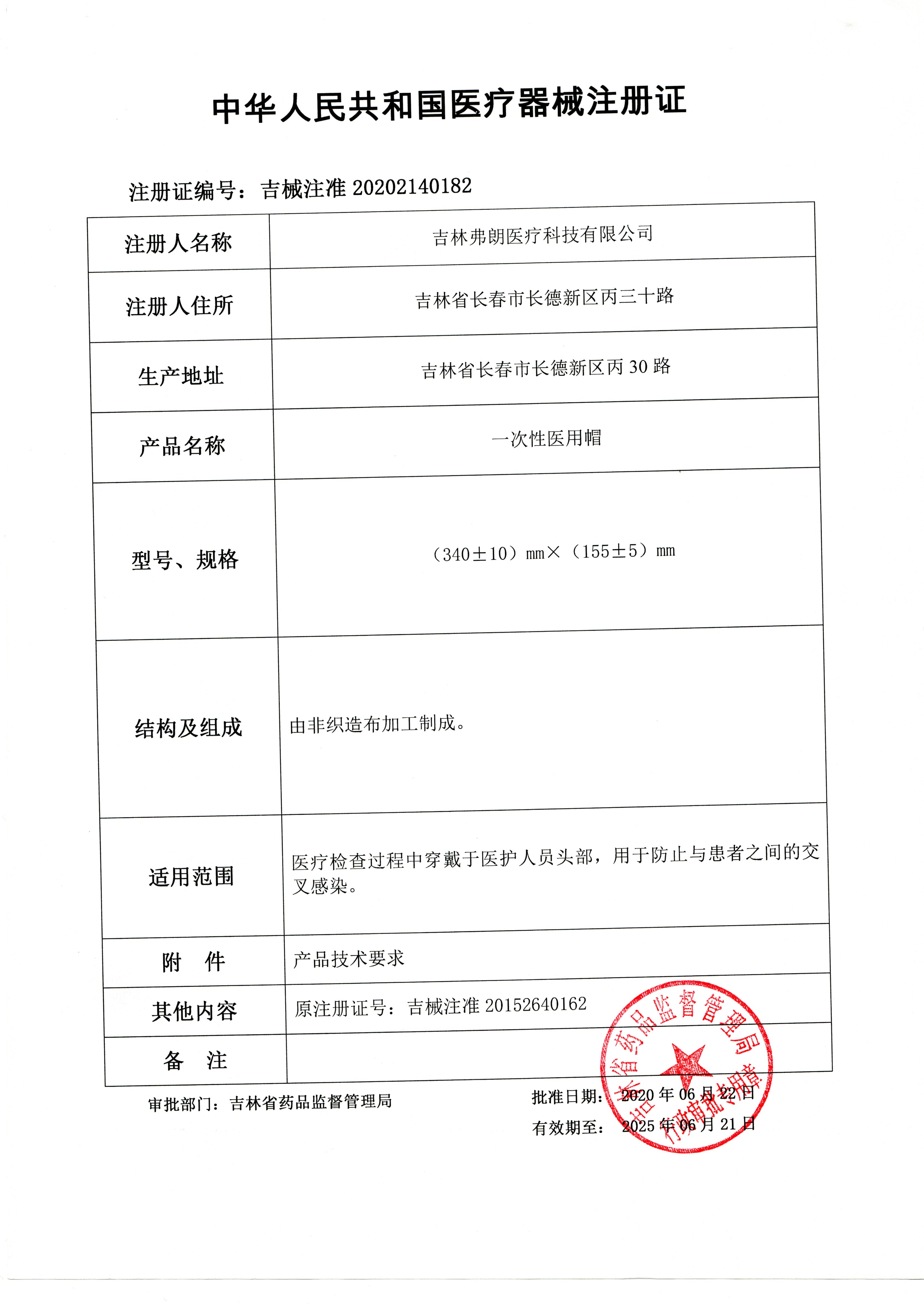
[Registrant/Company Name] Jilin Fran Medical Technology Co., Ltd
[Domicile] Bing30th Road, Changde New District, Changchun, Jilin Province
[Production address] Bing30 Road, Changde New District, Changchun, Jilin Province
[Contact Information] 0431-81851702
[Production License No.] Ji Shi Yao Jian Xi Production License No. 20150048
[Medical Device Registration Certificate No.] Ji Xi Zhu Zhun 20202140182
[Product Technical Requirements No.] Jixi Registration No. 20202140182
The main structure and composition of the product are made by processing non-woven fabrics.
[Product performance] 1. The surface of disposable medical caps should be clean and free from defects such as stains, mold spots, and damages; The connected parts should be firm, uniform, and straight, with neat formed edges and no defects such as skewness or breakage; The elastic band used should have good elongation and contraction.
2. The specification size is (340 ± 10) mm × (155 ± 5) mm
3. Performance requirements for disposable medical caps: According to the standard performance requirements for surgical gowns (non critical areas) in YYT 0506.2-2016 "Surgical Orders, Surgical gowns, and Clean Clothes for Patients, Medical Personnel, and Instruments"
4. The residual amount of ethylene oxide should not exceed 10 μ G/g
5. Sterile
[Product scope of application] Wear on the head of medical staff during medical examinations to prevent cross infection with patients.
[Contraindications] None.
[Precautions, Warnings, and Indicative Notes]
1. Before use, check the sterilization validity period first, and use beyond the validity period is strictly prohibited;
2. This product is sterilized and sterile with ethylene oxide, and it is prohibited to use if the packaging is damaged;
3. This product is disposable and will be disposed of in accordance with the Medical Waste Management Regulations after use;
4. Please refer to the certificate of conformity or minimum inner packaging for product specifications, models, quantities, production batch numbers, production dates, and sterilization dates.