-
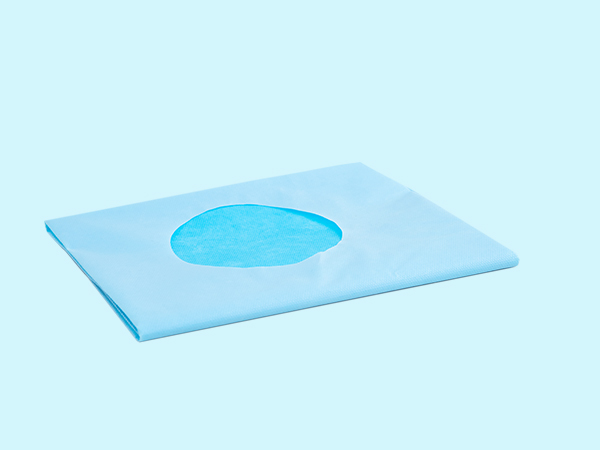
[Product Name] Disposable hole towel
[Registrant/Company Name] Jilin Fran Medical Technology Co., Ltd
[Domicile] Bing30th Road, Changde New District, Changchun, Jilin Province [Production address] Bing30th Road, Changde New District, Changchun, Jilin Province
[Contact Information] 0431-81851702 [Production License Number] Ji Shi Yao Jian Xi Production License No. 20150048
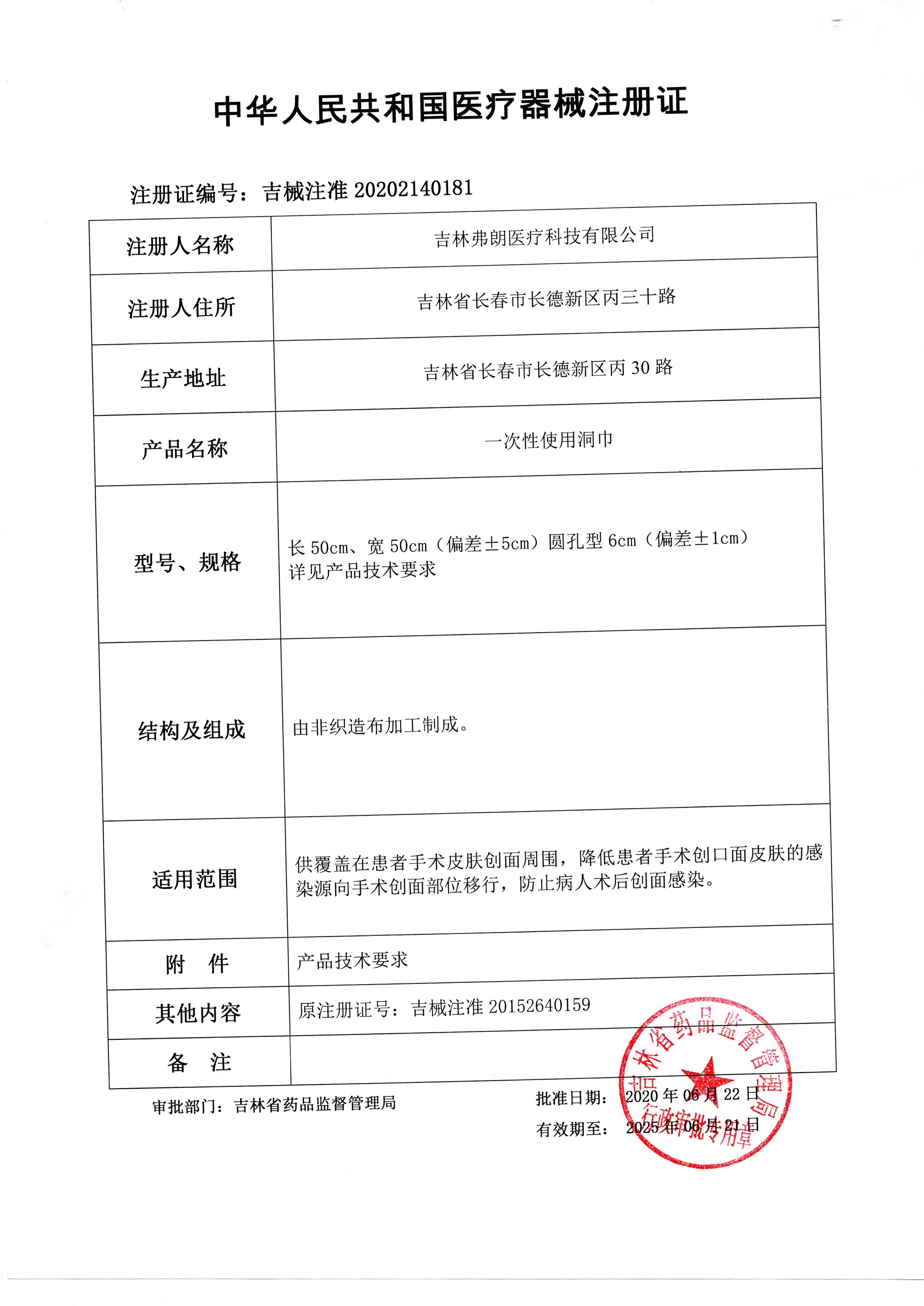
[Medical Device Registration Certificate No.] JiXZZ No. 20202140181 [Product Technical Requirements No.] JiXZZ No. 20202140181
The main structure and composition of the product are made by processing non-woven fabrics.
[Product Performance] 1. The specifications and dimensions of disposable hole towels shall comply with the requirements in Tables 1 and 2.
The surface of disposable hole towels should be clean, flat, and free from defects such as holes and stains; The opening should be uniform and the formed edges should be neat.
Performance requirements for disposable hole towels: According to YY/T 0506.2-2016 "Surgical sheets, surgical gowns, and clean clothing for patients, medical staff, and instruments"
Standards should meet the standard performance requirements of the surgical order (critical and non critical areas).
Note: The key area for disposable hole towels is the edge of the hole, while the rest are non critical areas.
The residual amount of ethylene oxide should not exceed 10 μ G/g.
Product sterility
[Scope of Application] Used to cover the patient's surgical skin around the wound surface, reducing the migration of infection sources from the surgical wound surface to the surgical wound site, and preventing postoperative wound infection.
[Contraindications] None
[Precautions, Warnings, and Indicative Notes]
Before use, first check whether the packaging is intact, and confirm the packaging label, production date, and sterilization validity period, and use it within the sterilization validity period;
This product is sterilized and sterile with ethylene oxide, and it is strictly prohibited to use if the packaging is damaged;
This product is disposable and should be disposed of in accordance with the Medical Waste Management Regulations after use. Reuse is prohibited;
Please refer to the certificate of conformity or minimum inner packaging for product specifications, models, quantities, production batch numbers, production dates, and sterilization dates.
✕