[Product Name] Disposable Pad
[Specification and Model] Type A: Small cushion Type B: Medium single
(Specific dimensions are as follows: small cushion: 30cm × 40cm, 40cm × 40cm, 40cm × 45cm, 40cm × 50cm, 40cm × 60cm, 50cm × 50cm, 50cm × 60cm, 60cm × 60cm, 70cm × 60cm, 70cm × 70cm, 70cm × 80cm, 70cm × 90cm, 70cm × 100cm, 80cm × 60cm, 80cm × 80cm, 80cm × 90cm, 80cm × 100cm, 90cm × 90cm, 90cm × 100cm, 100cm × 100cm
Middle order: 80cm × 120 cm, 80cm × 220 cm, 90 cm × 150 cm, 90cm × 220cm, 100cm × 120cm, 100cm × 130cm, 100cm × 140cm, 100cm × 150 cm, 100cm × 160cm, 100cm × 170cm, 100cm × 180cm, 100cm × 190cm, 100cm × 200cm, 100cm × 210cm, 100cm × 220cm, 110cm × 110cm, 180cm × 120cm, 200cm × 120cm, 200cm × 150cm, 120cm × 150cm)
Note: Special specifications and sizes can be customized according to customer requirements.
[Registrant/Company Name] Jilin Fran Medical Technology Co., Ltd
[Domicile] Bing30th Road, Changde New District, Changchun, Jilin Province
[Production address] Bing30 Road, Changde New District, Changchun, Jilin Province
[Contact Information] 0431-81851702
[Production License No.] Ji Shi Yao Jian Xi Production License No. 20150048
[Medical Device Registration Certificate No.] Ji Xi Zhu Zhun 20202140180
[Product Technical Requirements No.] Jixi Registration No. 20202140180
The main structure and composition of the product are made of non-woven fabric and soft polyvinyl chloride film thermally synthesized laminated fabric as raw materials, which are cut and processed. [Product performance] 1. The specifications and dimensions of disposable pads should comply with the requirements specified in technical requirement 1.2.
2. The appearance of disposable pads should be flat, clean, and free from defects such as stains, holes, wrinkles, etc; The formed edges should be neat, uniform, straight, and free from cracking.
3. Performance requirements for disposable pads: According to the standard performance requirements (key areas) for surgical sheets in YY/T 0506.2-2016 "Surgical sheets, surgical gowns, and clean clothing for patients, medical staff, and instruments".
4. The residual amount of ethylene oxide shall not exceed 10 μ G/g
5. Product sterility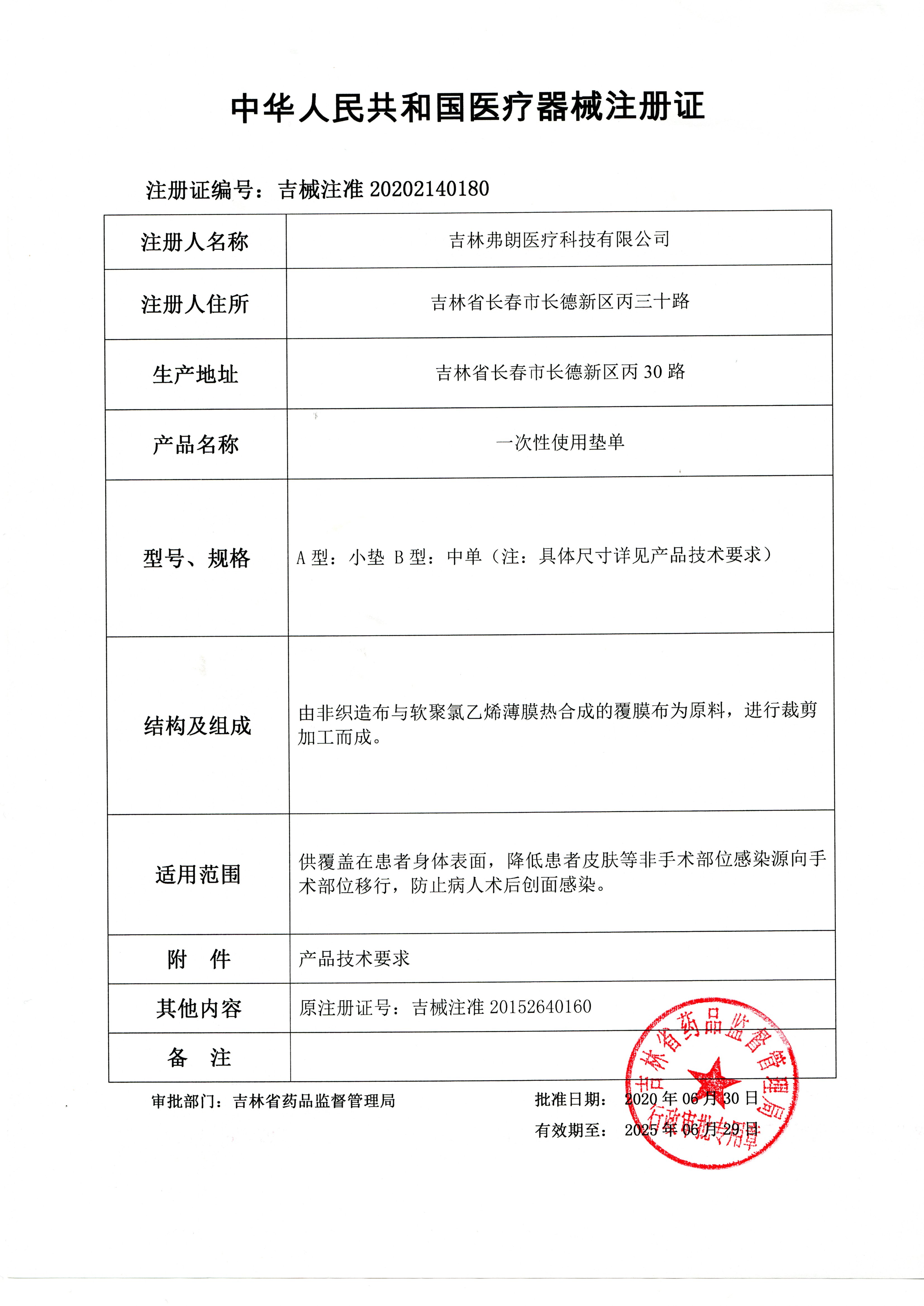 [禁忌症]无。
[禁忌症]无。
[注意事项、警示及提示性说明]
1 、使用前首先核对包装是否完好,并对包装标识、生产日期、灭菌有效期进行确认,并在灭菌有效期内使用; 2 、本产品经环氧乙烷灭菌无菌提供,包装破损严禁使用;
3 、本品为一次性使用,使用后按照《医疗废物管理条例》进行处理,禁止重复使用;
4 、产品规格、型号、数量、生产批号、生产日期、灭菌日期详见合格证或最小内包装。











