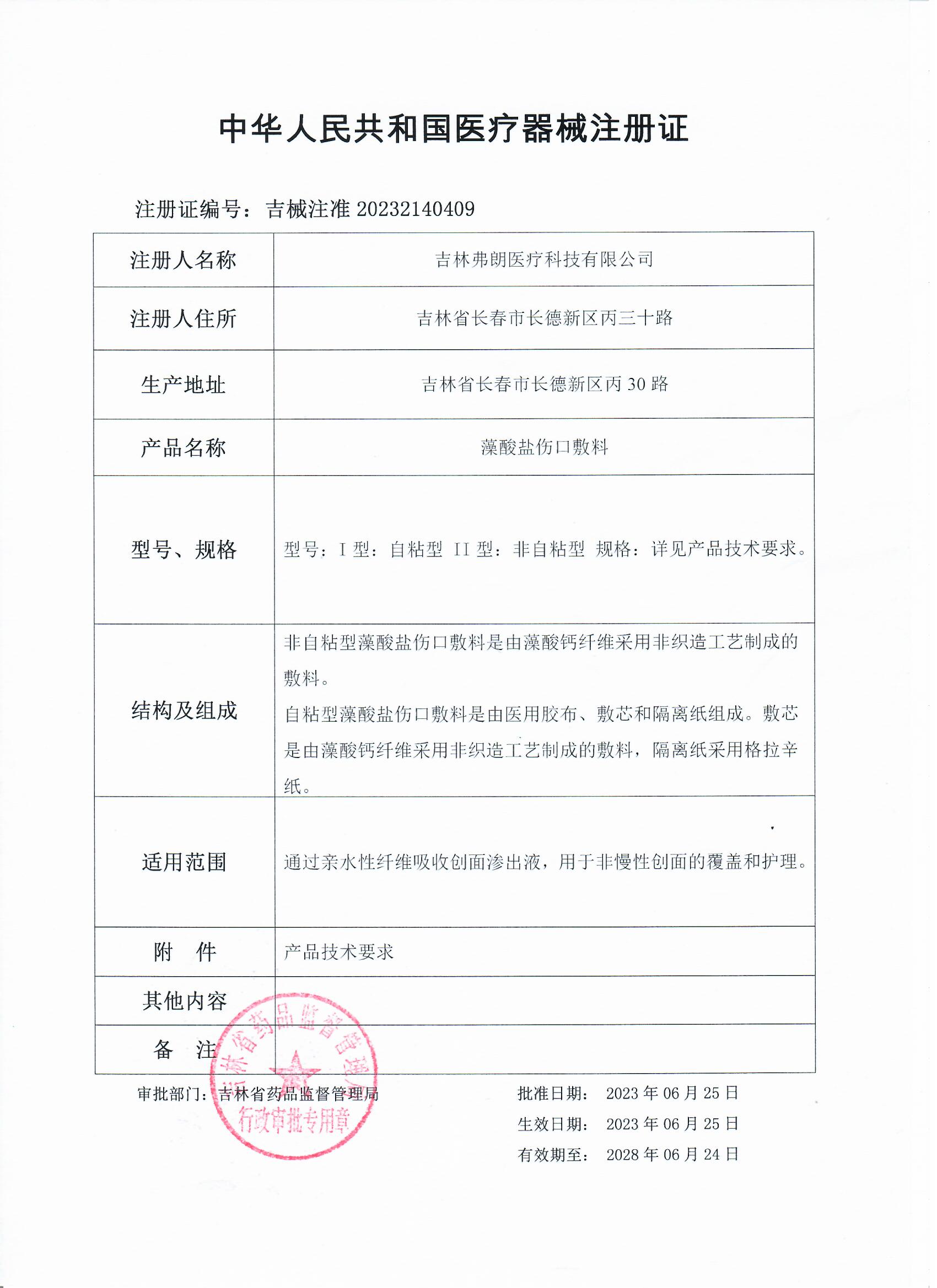
[Product Name] Alginate Wound Application
[Specification and Model] Model: Type I: Self adhesive Type II: Non self adhesive Type Specification: Please refer to the product technical requirements for details.
[Name of Registrant/Name of Production Enterprise] Jilin Fran Medical Technology Co., Ltd
[Registrant/Production Enterprise Domicile] Bing30th Road, Changde New District, Changchun, Jilin Province
[Production address] Bing30 Road, Changde New District, Changchun, Jilin Province
[Contact Information] 0431-81180431
[Production License No.] Jiyao Jianxi Production License No. 20150048
[Medical Device Registration Certificate No.] Ji Xi Zhu Zhun 20232140409
[Product Technical Requirements No.] Jixi Zhuzhuan 20232140409
[Structure and composition]
Non self adhesive alginate wound dressing is a dressing made from calcium alginate fibers using a non-woven process.
Self adhesive alginate wound dressing is composed of medical tape, core and isolation paper.
The core dressing is made of calcium alginate fibers using a non-woven process, and the isolation paper is made of grazin paper.
[Product performance]
Appearance: The surface should be flat, clean, and undamaged; Liquid absorption: should not be less than 10 times its initial weight; Heavy metal content (calculated in Pb): The heavy metal content should not exceed 20 μ G/g; Sterile: should be sterile; Please refer to the product technical requirements for other performance details.
[Scope of Application] Absorbs wound exudate through hydrophilic fibers for coverage and care of non chronic wounds.
[Instructions for Use]
Instructions for use of alginate wound dressing (Type I: self-adhesive):
1. Wound preparation: Clean the wound with physiological saline and gently dry the surrounding skin with sterile gauze; 2. Remove the application, peel off the isolation film, and do not touch the core of the application; 3. Fully cover the wound with the core application and apply medical tape to the skin around the wound, smoothing and compressing it; 4. Replace the application in a timely manner based on the amount of exudate from the wound.
Instructions for use of alginate wound dressing (type II: non self-adhesive):
Wound preparation: Clean the wound with physiological saline and gently dry the surrounding skin with sterile gauze; 2. Cover the surface of the wound directly with the application, and secondary dressing is required on the outside; Replace the application in a timely manner based on the amount of exudate from the wound.
[Contraindications] This product is not suitable for dry wounds, and is contraindicated for those with skin diseases or skin allergies.
[Precautions, Warnings, and Indicative Notes]
1. This product is a disposable product and repeated use is strictly prohibited.
2. Determine the frequency of replacement based on the condition of the wound and use it until the wound heals.
3. The self-adhesive application should ensure that the core part can fully cover the wound, and the medical tape part cannot directly contact the wound surface.
4. To treat infectious wounds, it is necessary to evaluate the patient's clinical symptoms before using this patch under the guidance of medical staff.
5. This product has been sterilized with ethylene oxide. If the inner packaging bag is damaged, please do not use or extinguish it